Thank you for Subscribing to Healthcare Business Review Weekly Brief
Be first to read the latest tech news, Industry Leader's Insights, and CIO interviews of medium and large enterprises exclusively from Healthcare Business Review
The Integration of Prosthetics and Orthotics in European Healthcare
By
Healthcare Business Review | Friday, March 28, 2025
Stay ahead of the industry with exclusive feature stories on the top companies, expert insights and the latest news delivered straight to your inbox. Subscribe today.
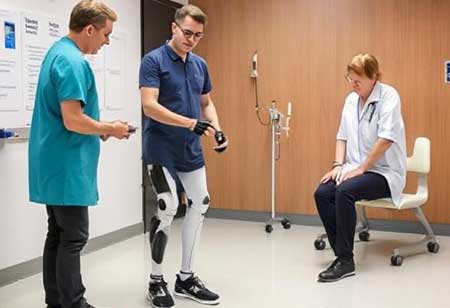
Prosthetics and orthotics (P&O) play a crucial role in European healthcare, continually advancing to enhance patient mobility and overall quality of life. As the demand for sophisticated assistive devices rises, healthcare systems increasingly rely on cutting-edge technology, innovative materials, and interdisciplinary medical expertise to meet patient needs.
Market Expansion and Industry Outlook in the European Market
The European prosthetics and orthotics sector has experienced consistent growth, driven by an aging population, a rise in diabetes and vascular diseases, and greater awareness of advanced technological solutions. Industry projections indicate substantial market expansion in the coming years, reinforcing healthcare service delivery across the continent.
Educational initiatives, advocacy for timely access to assistive care, and standardising healthcare practices within the P&O field further support this growth. Additionally, European governments have strengthened healthcare budgets to accommodate the evolving needs of patients who rely on these essential devices.
Technological Innovation in Prosthetics and Orthotics
Europe is at the forefront of developing advanced prosthetic and orthotic solutions. Integrating artificial intelligence, robotics, and 3D printing has created lighter, more durable, and highly functional devices.
Smart prosthetics, equipped with embedded sensors and microprocessors, enable precise movement control and seamless interaction with the user’s body. Similarly, 3D-printed orthotic devices provide cost-efficient, custom-fitted solutions that enhance user comfort and mobility.
Advancements in material science, including using carbon fiber composites and thermoplastics, have further improved design flexibility, durability, and anatomical accuracy. These innovations continue to shape the future of P&O, offering highly personalised and effective solutions for patients.
Integration into European Healthcare Systems
P&O care is seamlessly integrated into European healthcare systems, essential to rehabilitation and long-term patient management. Holistic care, which includes biomechanical assessments, physical therapy, and psychological support, benefits individuals with congenital conditions, traumatic injuries, or degenerative diseases.
The structured nature of European healthcare systems ensures equitable access to P&O services through comprehensive insurance coverage and coordinated medical teams. These networks facilitate streamlined processes from diagnosis to device fitting and ongoing maintenance, enhancing patient satisfaction and long-term outcomes.
The Role of Interdisciplinary Collaboration
Effective prosthetic and orthotic care relies on interdisciplinary collaboration among orthotists, physiatrists, orthopedic surgeons, and physical therapists. This collective expertise ensures that patients receive individualised treatment plans tailored to their unique medical and functional needs.
Incorporating rehabilitation specialists into patient care enhances device usability and mitigates the risk of secondary complications. Furthermore, interdisciplinary cooperation fosters continuous innovation in device performance and therapeutic outcomes, driving progress in the field.
Patient-Centered Design and Customization
The European P&O industry prioritises patient-centered design, emphasising comfort, personalisation, and functionality. Patients are actively engaged in prosthetic and orthotic device design and testing phases, increasing acceptance, adherence, and long-term usage.
Healthcare institutions also leverage data analytics to refine device designs based on user feedback, mobility patterns, and ergonomic considerations. These efforts ensure that assistive devices are adaptable to the diverse needs of individual patients, further optimising their effectiveness.
Education and Professional Development
Sustaining growth in the P&O sector requires a strong emphasis on education and professional training. Many European countries have introduced specialised certification programs and clinical training to uphold high prosthetic and orthotic care standards.
Universities and healthcare institutions collaborate with research organisations to develop cutting-edge solutions that enhance patient care. Public health campaigns also play a key role in raising awareness about the availability and benefits of prosthetic and orthotic interventions.
Regulatory Standards and Compliance
The European P&O industry operates within a stringent regulatory framework to ensure the safety and efficacy of assistive devices. Regulatory bodies, including the European Medicines Agency (EMA), oversee the evaluation and certification of prosthetic and orthotic products.
These regulations mandate rigorous testing, clinical trials, and material validation, fostering trust and confidence among healthcare professionals and patients. Transparency in regulatory processes strengthens service delivery and maintains high-quality standards across the sector.
Commitment to Inclusivity and Accessibility
Inclusivity remains a cornerstone of the European P&O industry. There is a strong focus on developing assistive devices tailored to diverse patient groups, including children, older adults, and athletes with specialised needs. This commitment ensures that many users can access high-quality, functional solutions.
Furthermore, government subsidies and inclusive workplace policies support individuals from low-income backgrounds who require prosthetic or orthotic interventions. These initiatives enhance accessibility and promote equitable healthcare solutions for all patients.
Key Trends Shaping the Future of P&O
One significant development is the extended use of wearable technology, integrating smart devices into prosthetics to monitor health metrics and enhance performance. Sustainability is also gaining prominence, with a growing emphasis on developing eco-friendly and recyclable materials for device production. Additionally, advancements in machine learning are driving hyper-personalized solutions by predicting patient needs and optimising device customisation. These innovations position Europe at the forefront of P&O advancements, offering improved functionality, greater comfort, and an enhanced quality of life for individuals relying on these technologies.
P&O constitute a vital and evolving component of European healthcare. Continuous innovation, interdisciplinary collaboration, and patient-centered approaches are redefining the integration of assistive technology within medical care. A steadfast commitment to inclusivity, advanced design, and regulatory excellence reinforces Europe’s leadership in delivering transformative solutions that enhance quality of life.